By ACRO AI/ML Committee Members:
Tala Fakhouri, VP, AI & Digital Policy, Real-World Research, Parexel International
Stephen Pyke, Chief Clinical Data & Digital Officer, Parexel International
The White House’s new AI Action Plan is a bold statement of U.S. ambition to lead globally in artificial intelligence (AI) innovation. It lays out a comprehensive strategy to accelerate adoption and deployment across critical sectors including healthcare, energy, and manufacturing. While not tailored specifically for the life sciences sector, its provisions on regulation, data infrastructure, and scientific enablement have clear implications for clinical research organizations (CROs), clinical technology organizations (CTOs), and pharmaceutical sponsors. For our industry, this is not just a policy document; it is a signal to engage, experiment, and lead.
With the Action Plan’s endorsement of AI’s role to “discover new materials, synthesize new chemicals, manufacture new drugs”, it recognizes the central role of AI in biomedical innovation. This blog explores what the Action Plan could mean for our industry and outlines how ACRO believes we can responsibly harness this momentum to accelerate the development of safe and effective drugs.
Enabling AI Adoption Through Smarter Regulation
According to the Action Plan, a major barrier to harnessing AI’s transformative potential is not the availability of AI-enabled technologies, but rather their limited use in established sectors. For healthcare, the limited scaled adoption of AI is partly due to challenges like regulatory uncertainty, lack of trust in AI-derived outputs, and limited access to “good quality” data needed to train, tune, and test AI-enabled technologies. To address this, the Action Plan directs agencies to review and revise regulations that unnecessarily hinder AI innovation, aligning with Executive Order 14192 on deregulation. It is unclear if the newly published draft FDA guidance documents for drugs and medical devices will be impacted. These draft guidance documents have been largely viewed by industry as positive innovation enabling documents, and the FDA is currently reviewing public comments to move them along from draft to final.
Crucially, the White House AI Action Plan recommends the establishment of regulatory sandboxes and AI Centers of Excellence, where tools can be tested in real-world settings under flexible regulatory supervision. Although the details are not yet known, for drug development, this could allow companies to pilot AI-enabled technologies for protocol optimization, trial monitoring, digital twins, AI-derived biomarkers and outcomes, or pharmacovigilance in a controlled and collaborative testing environment with regulators. Such sandboxes, enabled by agencies like the FDA, would encourage iterative learning and shared insights. Additionally, it is possible that these sandboxes can be used to accelerate the qualification of innovative AI methods and tools under the drug development tools program at the FDA, which has been criticized for being slow and unpredictable.
ACRO supports this direction and encourages the FDA to launch a drug development–specific sandbox for AI-enabled technologies that can help accelerate the development of safe and effective drugs for patients. ACRO also urges that participation be open to CROs, given that CROs handle more clinical trial data than any other participants (i.e., biotechnology and pharmaceutical companies) in our ecosystem.
From Caution to Confidence: Building AI Standards
The Action Plan charges the National Institute of Standards and Technology (NIST) with leading domain-specific AI standards, including in healthcare, and doing so with broad input from public, private, and academic stakeholders.
This is especially important for AI uses that influence regulatory decision-making in drug approvals (e.g., AI models used for trial eligibility, extraction of unstructured data from EHRs for patient selection, digital twins for placebo simulations, endpoint prediction, and real-time safety signal detection). For such uses, validation benchmarks and clear performance thresholds that are tailored to the specific context of use are essential. ACRO encourages alignment of such standards with the FDA’s risk-based credibility assessment framework, which provides a structured approach for evaluating the reliability of AI-derived evidence.
Importantly, the plan also supports the establishment of reference datasets and shared evaluation methods, which are fundamental to reproducibility. Friends of Cancer Research and others have emphasized that benchmarks and high-quality public datasets are foundational to safe and scalable AI. We agree. ACRO recommends the creation of FDA-endorsed reference datasets and performance metrics, especially in oncology and rare disease research, to guide AI model development and evaluation. Importantly, ensuring that these data are representative of the intended patient population will be critical.
High-Quality Data: A Strategic Asset
A key area of focus from the Action Plan is on the availability of “good data”. The plan proposes robust actions to create the world’s highest-quality scientific datasets, particularly in biology, chemistry, and medicine.
Agencies like the National Science Foundation (NSF) and the Department of Energy (DOE) are tasked with building secure computing environments and expanding federally accessible datasets. The plan would make data from NIH, CMS, CDC, FDA, and others more readily available through the National Secure Data Service (NSDS), provided privacy and confidentiality are protected. It is unclear how sponsor-owned commercially confidential information and trade secrets will be maintained and secured, and gaining traction within the biopharmaceutical industry would require clear guardrails in this area.
The emphasis on access to high-quality data presents a significant opportunity for AI-driven research. CROs and sponsors could potentially be able access rich multimodal data including de-identified real-world data (e.g., EHR and claims data), genomic libraries, or clinical trial registries in secure enclaves to develop robust models that can be used for a variety of reasons including developing external control arm studies, patient selection for trials, and for precision medicine initiatives.
We encourage the administration to ensure that biomedical researchers in our industry, not just academia, can participate in these secure data initiatives. Industry has access to highly curated datasets collected using robust protocols, and our involvement in such initiatives can prove beneficial to all. Further, it is critical that data made available to train, tune, and test AI-enabled technologies are representative of the intended patient population, not just within the U.S. but also globally. Last, ACRO recommends that U.S. efforts to curate AI-ready data consider the utility of a federated learning model, where models learn across institutions without centralizing data. This may be critical for securing patient privacy and protecting intellectual property.
Scaling Science with AI-Powered Labs
The Action Plan also addresses a practical bottleneck: AI-driven predictions are only valuable if they can be tested quickly and at scale. It calls for the creation of automated, cloud-enabled laboratories that pair AI models with robotic experimentation.
This model, already emerging in “self-driving labs” for chemistry and biology, could transform drug discovery. Industry can synthesize and test AI-suggested compounds far more efficiently, while reducing time and human error.
We recommend NIH, FDA, and other agencies consider public-private collaborations with broad representation (i.e., academia, sponsors, CROs, CTOs, etc.) to develop these AI-enabled labs. These broad partnerships can bring together funding, infrastructure, regulatory expertise, and operational excellence—to achieve hard-to-reach scientific goals.
Cultural Change: AI as a Partner, Not a Threat
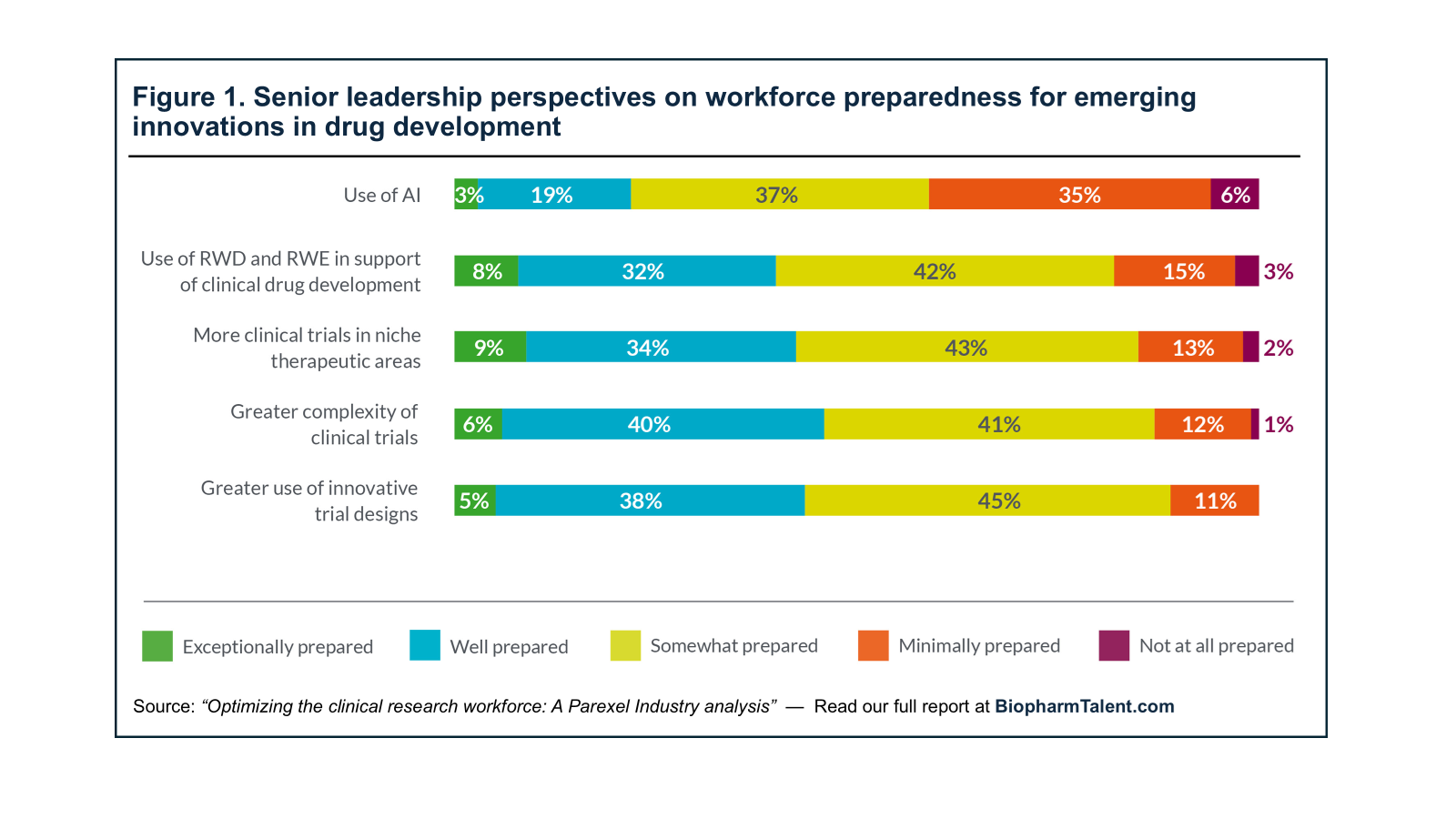
The Action Plan promotes a “try-first” mindset, where experimentation with AI is rewarded, and slow adoption is no longer acceptable. But this cultural shift will only succeed in healthcare if the workforce feels empowered and equipped to engage with AI tools, and if the workforce “trusts” the outputs from these tools. This shift is vital. Industry leaders must support staff in overcoming internal hesitations about AI and invest in upskilling their workforce, training teams to responsibly design, validate, and deploy AI across organizations. This was also recently highlighted by a survey of industry leaders that was conducted by Parexel. According to the 2025 survey, biopharmaceutical industry leaders did not think the current workforce is fully prepared with the necessary skills in key innovative areas such as the use of AI (see Figure 1). As the White House AI Action Plan emphasizes, AI will complement human expertise—not replace it. The future of drug development lies in augmented intelligence, not artificial intelligence alone.
What Should CROs, CTOs, and Pharma Do Now?
1. Engage Early with Sandboxes and Pilots
Prepare for participation in FDA-led or cross-agency AI regulatory sandboxes. These will be critical in shaping regulatory expectations and ensuring your AI-enabled technologies are evaluated appropriately.
2. Align with Standards Efforts
Track and contribute to NIST-led standards initiatives. Ensure your tools are validated against emerging national metrics, and document performance and limitations clearly.
3. Leverage Public Data Securely
Explore collaborations through NSDS or other secure data environments. Look for opportunities to augment your own data with high-quality, privacy-protected government datasets.
4. Adopt a Good AI Practices Framework
Just as GCP and GMP guide other areas of drug development, begin implementing internal practices to ensure model transparency, version control, and reproducibility.
5. Invest in People
Develop internal AI literacy. Train cross-functional teams to work effectively with machine learning models in trial design, execution, and analysis.
A Vision for Patients
Ultimately, the value of the White House AI Action Plan will be measured by its impact on patients. If implemented thoughtfully, this framework could help accelerate the development of safe and effective drugs, reduce trial timelines, and broaden access to clinical research by improving recruitment and tailoring interventions. Consistent and predictable regulations, better predictive models, faster experimentation, and higher quality data can lead to a drug development enterprise that is faster, smarter, and more equitable – delivering innovation that reaches patients when they need it the most.
ACRO and our members stand ready to lead in this new chapter of biomedical innovation.
