The U.S. biopharmaceutical industry leads globally in the development of new medicines and remains at the forefront of scientific and technological advances that promise to deliver new treatments and improve health outcomes. Developing and evaluating these drugs requires significant investments—such as securing trial sites, hiring staff and contractors, recruiting, and treating trial volunteers—all of which drive considerable economic spending in the U.S. and abroad. U.S.-based biopharmaceutical companies account for half of all U.S. investments in biomedical research and development, including significant spending in clinical research.1 In fact, in 2023, biopharmaceutical company investments in U.S. clinical trial sites alone generated more than $62 billion in economic activity.2
However, the drug discovery and development process is extremely time-consuming and expensive. The clinical stage alone costs $117.4 million and makes up over 68% of total research and development expenditure.3 Biopharmaceutical companies commonly rely on outsourcing to clinical research organizations (CROs) to shorten product development time and cut fixed overhead costs, with nearly 75% of all clinical trials outsourced to CROs.4 Together with clinical technology organizations (CTOs), these entities play a critical role in the drug development process and have a positive impact on both the U.S. and global economy. ACRO member companies support or conduct a majority of industry-sponsored clinical trials worldwide. In 2024, ACRO members operated in every global region and generated an estimated $98 billion in revenue.5
The CRO/CTO market is undergoing a period of rapid growth and transformation, both domestically and abroad, driven by increases in the number of clinical trials, optimization of drug development pipelines, rising research and development spending, and the integration of digital tools. The global CRO market has steadily grown to meet the demand for safer and more cost-effective drug development, with a market size increase of more than $6 billion, from $59 billion in 2024 to $65 billion in 2025. By 2029, the CRO market is projected to grow to $94.61 billion.6 Correspondingly, a study by Nova One Advisor found that the global clinical trial technology and services (CTO) market has also grown to meet the increased complexity of trials, regulatory demands, and the ever-growing volume of data. From 2024 to 2025, the CTO market increased by $4 billion from $26.15 billion to $30.20 billion. By 2029, the CRO market is projected to grow to $53.74 billion.7
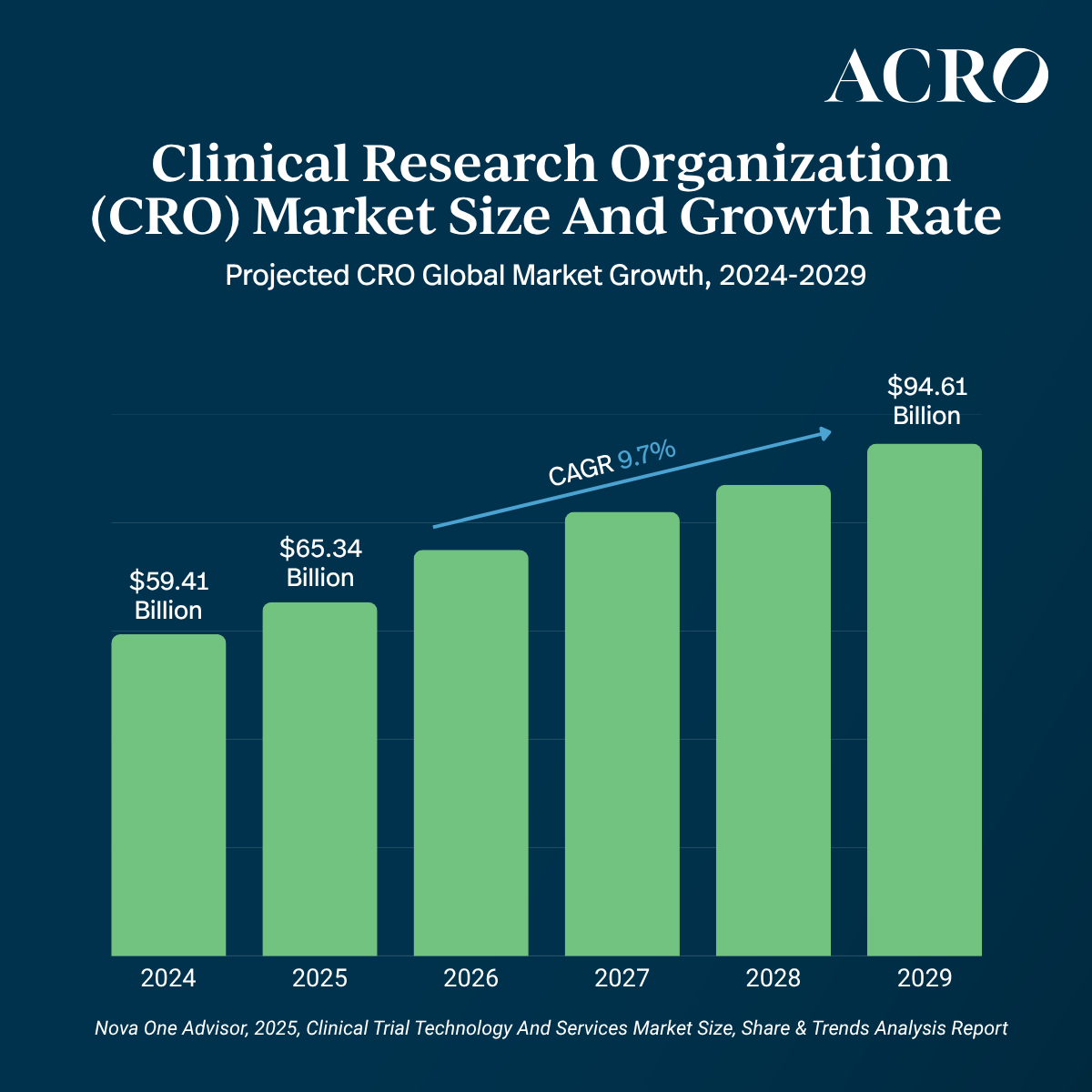
These projections describe the vital role that CROs and CTOs play in the biopharmaceutical ecosystem, not only accelerating the development of lifesaving therapies but also delivering meaningful economic benefits both in the U.S. and globally.
Footnotes
- Research!America, U.S. Investments in Medical and Health Research and Development: 2016 – 2020, January 2022. ↩︎
- TEConomy Partners, Biopharmaceutical Industry-Sponsored Clinical Trials: Impacting State Economies, March 2025. ↩︎
- Eastern Research Group, Inc. submitted to Assistant Secretary for Planning and Evaluation (ASPE), Drug Development Report, September 2024. ↩︎
- Straights Research, Clinical Trials Outsourcing Market Size, Share & Growth Report by 2033, September 2024. ↩︎
- “ACRO 2024 Member Demographics Survey.” ACRO, Association for Clinical research Organizations (ACRO), 22 May 2025, www.acrohealth.org/acro-member-demographics-survey/. ↩︎
- The Business Research Company, 2025, Clinical Research Organization Global Market Report 2025, https://www.thebusinessresearchcompany.com/report/clinical-research-organization-global-market-report. Accessed 2 July 2025. ↩︎
- Nova One Advisor, 2025, Clinical Trial Technology And Services Market Size, Share & Trends Analysis Report, https://www.novaoneadvisor.com/report/clinical-trial-technology-and-services-market. Accessed 2 July 2025. ↩︎
