ACRO member companies have compiled a series of case studies that demonstrate how centralized monitoring identifies critical issues more quickly and effectively than traditional on-site monitoring methods. This is the fourth post in a series highlighting centralized monitoring at work. Check back for more examples of how centralized monitoring has benefited ACRO member companies and their sponsor partners.
Case Study 1: Catching Equipment Issues Early
A data surveillance plan, including key risk indicators (KRIs), quality tolerance limits (QTLs), and aggregated data analysis, was deployed as part of centralized monitoring deliverables for a Phase I oncology study. Electrocardiogram (ECG) variables were utilized as KRIs for safety and tolerability for the entire study duration. However, centralized monitoring indicated that 10% of patients at a specific site were missing a triplicate ECG, the practice of recording three separate ECGs within a short timeframe.
Further investigation revealed that the missed triplicate ECGs were due to an equipment malfunction at the site. As a result of the central monitor’s analysis and identification of the equipment malfunction, corrective actions were taken. The equipment vendor replaced the malfunctioning equipment, and the study site retrained on protocol compliance and the need for timely communication of support needs.
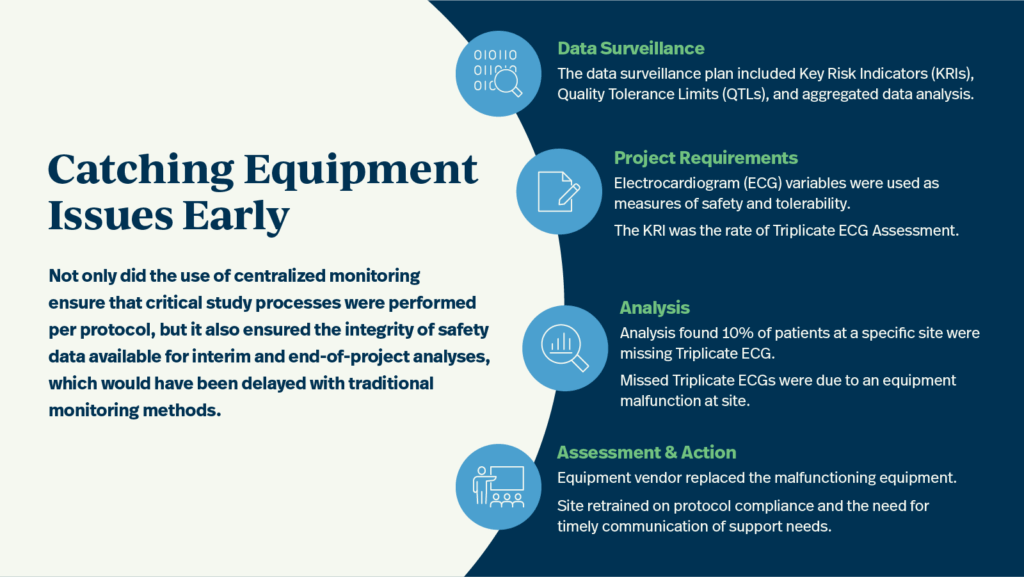
Not only did the use of centralized monitoring ensure that critical study processes were performed per protocol, but it also ensured the integrity of safety data available for interim and end of project analyses, which would have been delayed with traditional monitoring methods.
Case Study 2: Ensuring Data Quality Through Retraining
A data surveillance plan, including key risk indicators (KRIs), quality tolerance limits (QTLs), and aggregated data analysis, was deployed as part of the centralized monitoring deliverables for a Phase III rheumatology & immunology study. Psoriasis Area and Severity Index (PASI) and Investigator’s Global Assessment (IGA) were utilized as KRIs for patient safety and data integrity for the entire study duration. However, centralized monitoring indicated that 36% of randomized patients’ PASI and IGA scores were inconsistent across the project.
Further investigation by the project manager confirmed unexpected PASI/IGA inconsistency across multiple patient visits. The clinical research associate (CRA) found that different individuals were performing the PASI and IGA at multiple sites. As a result of the central monitor’s analysis and identification of assessor inconsistencies across patient visits and study sites, corrective actions were taken much earlier in the project’s lifecycle than would have been possible without central monitoring. Site staff were retrained on the application of PASI as well as on the relationship between PASI and IGA.
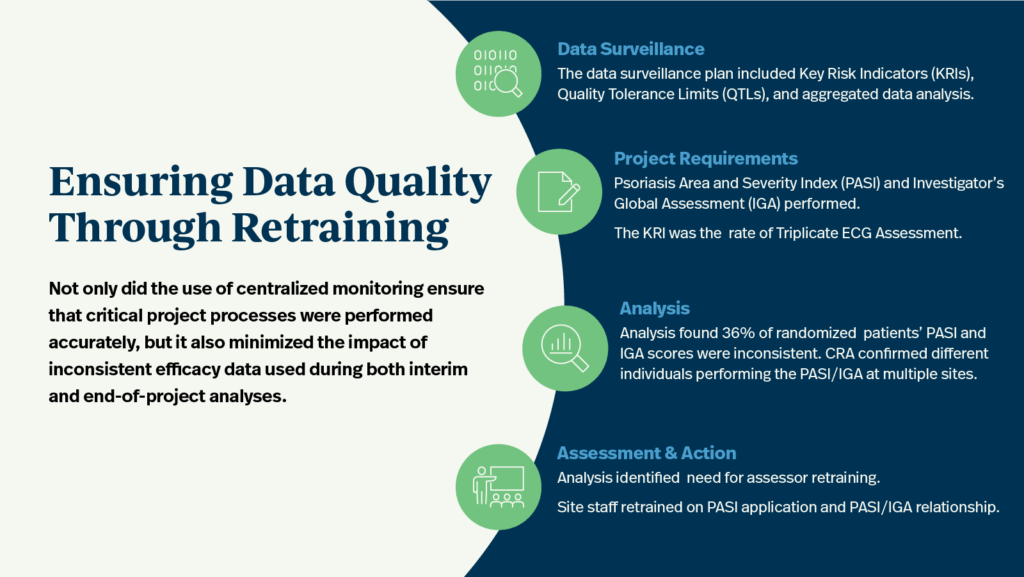
Not only did the use of centralized monitoring ensure that critical project processes were performed accurately, but it also minimized the impact of inconsistent efficacy data used during both interim and end-of-project analyses.
